Patient Resources
Get Healthy!
Results for search "Drug Approvals".
Health News Results - 21
FDA Moves Toward Faster Drug Approvals
- Deanna Neff HealthDay Reporter
- February 20, 2026
- Full Page
In a major shift that could fundamentally change how new medicine reaches the pharmacy, the U.S. Food and Drug Administration (FDA) is relaxing a long-standing drug approval requirement for common diseases.
Drugmakers must often complete two separate, large-scale studies...
Drugs Rarely Become Available In Lower-Income Countries Where They're Tested, Study Says
- Dennis Thompson HealthDay Reporter
- November 18, 2025
- Full Page
Pharmaceutical companies are using the citizens of lower-income countries as guinea pigs to test cutting-edge drugs headed mainly for the United States and other well-off nations, a new study says.
Only a quarter of medicines tested in other countries wound up available ...
Dr. Richard Pazdur Appointed to Lead FDA’s Drug Division After Turbulent Year
- I. Edwards HealthDay Reporter
- November 13, 2025
- Full Page
The U.S. Food and Drug Administration (FDA) has appointed one of its most respected cancer drug regulators to lead the agency’s main division for approving new drugs.
The appointment of Dr. Ric...
New Nonhormonal Drug Approved to Treat Menopause Symptoms
- Deanna Neff HealthDay Reporter
- October 27, 2025
- Full Page
The U.S. Food and Drug Administration (FDA) has approved a new nonhormonal treatment to help women manage menopause symptoms such as hot flashes and nig...
Eli Lilly to Buy Gene Therapy Firm Verve in $1 Billion Deal to Develop Heart Drug
- HealthDay Reporter
- I. Edwards
- June 18, 2025
- Full Page
Drugmaker Eli Lilly plans to buy Verve Therapeutics, a gene-editing startup, for about $1 billion upfront.
The deal gives Lilly a potential new treatment for heart disease, The Wall Str...
Texas Invests $50M in Psychedelic Drug Research to Treat Addiction
- HealthDay Reporter
- I. Edwards
- June 16, 2025
- Full Page
Texas has moved to fund research into ibogaine, a psychedelic drug that may help treat addiction, depression and brain injuries.
Gov. Greg Abbott signed a bill last week approving $50...
FDA Delays Final Approval of Novavax COVID Vaccine
- HealthDay Reporter
- I. Edwards
- April 4, 2025
- Full Page
The U.S. Food and Drug Administration (FDA) has delayed the full approval of Novavax’s COVID-19 vaccine.
The decision had been expected by April 1, but the agency now sa...
FDA OKs Amvuttra To Treat Heart Conditions
- HealthDay Reporter
- I. Edwards
- March 24, 2025
- Full Page
The U.S. Food and Drug Administration (FDA) has approved a new drug for a serious heart condition that affects thousands of people.
Experimental Alzheimer's Drug Slows Thinking Declines in Late-Stage Trial
- HealthDay Reporter
- Robin Foster
- July 17, 2023
- Full Page
Another experimental drug meant to slow the damage of Alzheimer's appears poised to join a growing arsenal of new treatments for this memory-robbing disease.
FDA Gives Full Approval to Alzheimer's Drug Leqembi
- HealthDay Reporter
- Robin Foster
- July 6, 2023
- Full Page
The U.S. Food and Drug Administration on Thursday gave full approval to the Alzheimer's drug Leqembi, clearing the way for insurance coverage of the pricey drug.
"The full FDA approval will open the floodgates for people with early Alzheimer's to get this drug. It's a bi...
FDA Approves New Drugs to Treat Type 2 Diabetes in Kids
- HealthDay Reporter
- Cara Murez
- June 21, 2023
- Full Page
The U.S. Food and Drug Administration on Tuesday approved two drugs that have been used in adults with type 2 diabetes for years for use in children aged 10 and up.
The approvals of Jardiance (empagliflozin) and Synjardy (empagliflozin and metformin hydrochloride) provid...
FDA Approves First Pill to Treat Moderate-to-Severe Crohn's Disease
- HealthDay Reporter
- Cara Murez
- May 19, 2023
- Full Page
Patients with Crohn's disease have a new treatment option, following U.S. Food and Drug Administration approval of a pill called Rinvoq (upadacitinib).
Rinvoq is meant to treat adults with moderately to severely active Crohn's disease who have not had success with TNF (t...
FDA Approves First Drug Meant to Ease Alzheimer's-Linked Agitation
- HealthDay Reporter
- Cara Murez
- May 11, 2023
- Full Page
A medication to treat agitation in Alzheimer's patients now has approval from the U.S. Food and Drug Administration.
The FDA gave supplemental approval to Otsuka Pharmaceutical Company Ltd., and Lundbeck Inc. for Rexulti (brexpiprazole) oral tablets on Thursday. Rexulti ...
Experimental Alzheimer's Drug Slows Decline in Thinking in Late-Stage Trial
- HealthDay Reporter
- Cara Murez
- May 3, 2023
- Full Page
Another experimental drug meant for Alzheimer's disease looks so promising that drugmaker Eli Lilly plans to ask the U.S. Food and Drug Administration for full approval by the end of June.
Known as donanemab, the medication clears amyloid plaque from the brain. In a lat...
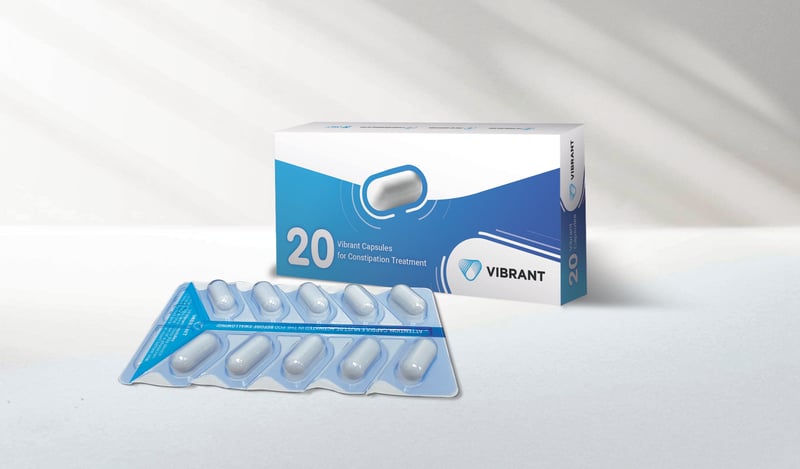
Could a Vibrating Pill Ease Chronic Constipation?
- HealthDay Reporter
- Cara Murez
- February 9, 2023
- Full Page
A new treatment for chronic constipation may bring relief without having to use drugs.
It's a vibrating pill called Vibrant that stimulates the colon as it passes through the body.
FDA Approves New 2-Drug Combo Medicine for Asthma
- HealthDay Reporter
- Cara Murez
- January 12, 2023
- Full Page
Adults with asthma now have a new rescue medication to turn to after the U.S. Food and Drug Administration approved Airsupra on Wednesday.
The drug is the f...
First FDA-Approved Fecal-Based Treatment Helps Fight a Tough Superbug
- HealthDay Reporter
- Cara Murez
- December 1, 2022
- Full Page
The U.S. Food and Drug Administration on Wednesday approved the first fecal microbiota treatment, aimed at helping adults battling tough-to-treat Clostridium difficile (C. diff) infections.
"Today's approval of Rebyota is an advance in caring f...
A Gene Therapy for Hemophilia That Costs $3.5 Million Gets FDA Approval
- HealthDay Reporter
- Cara Murez
- November 23, 2022
- Full Page
People with one form of the genetic blood disorder hemophilia now have a one-time treatment with a $3.5 million price tag.
The U.S. Food and Drug Administration approved the new gene therapy Hemgenix on Nov. 22. Soon after, drugmaker CSL Behring revealed its cost.
FDA Approves New ALS Drug Despite Uncertain Data
- HealthDay Reporter
- Ernie Mundell
- September 30, 2022
- Full Page
The U.S. Food and Drug Administration on Thursday gave its approval to a new drug for
Hints That Experimental Drug Might Curb a Form of ALS
- HealthDay Reporter
- Cara Murez
- September 23, 2022
- Full Page
People with a rare genetic form of ALS may benefit from extended use of an investigational drug, a new study shows.
The medication, tofersen, benefited patients with mutations of the gene SOD1. These mutations create a misfolded version of a protein, which leads to
Merck's COVID Pill Appears Effective, But May Pose Pregnancy Risks: FDA
- HealthDay Reporter
- Robert Preidt and Robin Foster
- November 29, 2021
- Full Page
Merck's experimental COVID-19 antiviral pill appears effective, but may pose risks for pregnant women, including birth defects and toxicity to developing fetuses, according to the U.S. Food and Drug Administration.